A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. 2015 AP Chemistry free response 3a.

What Is Double Replacement Reaction Example Share Education
Introduction to Scientific Experimentation - Students will be able to research and gain background information to make educated guesses and think critically about results and outcomes.

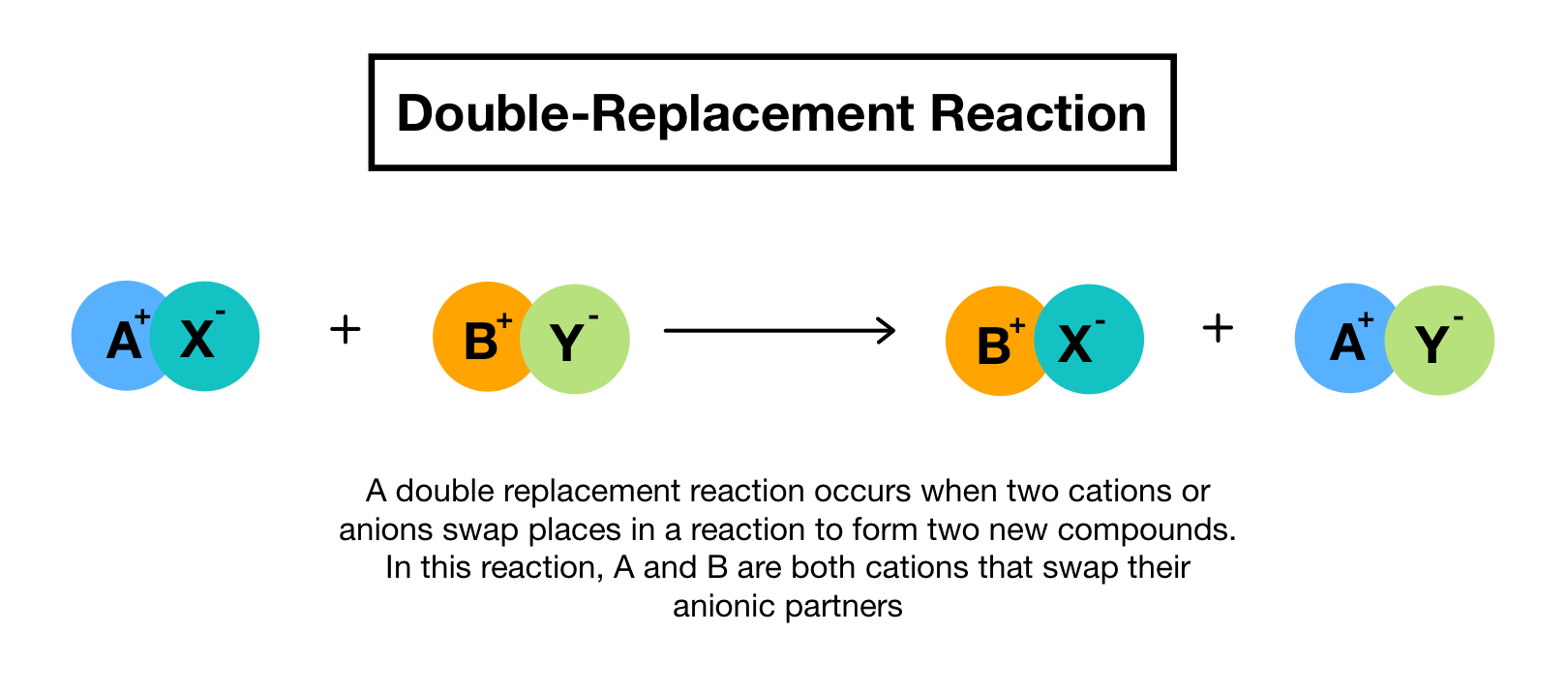
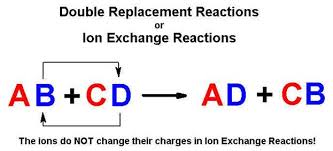
. A double replacement reaction is called a double decomposition reaction but the term is reserved for when one or both of the reactants doesnt dissolve in a solvent. Single replacement reaction. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
Double Replacement Reaction Examples. A double displacement reaction is a type of reaction in which two reactants exchange ions to form two new compounds. Lets consider the saturated solution of silver.
Get smarter in Chemistry on Socratic. This is the currently selected item. State the reaction in chemical formulas and in symbols.
In the same way for a single-displacement reaction an element can only be replaced if. The original electron from X that was participating in the shared bond with Y is donated to Y. Usually in these reactions when combining aqueous solutions a solid product is also formed.
Two of the compounds are reactants and 2 are products. Molecular complete ionic and net ionic equations. Single replacement reaction.
CuOH 2 2 HC 2 H 3 O 2-- CuC 2 H 3 O 2 2 2 H 2 O. A double replacement reaction will occur if a formation of a precipitate gas or water takes place. A double replacement reaction is represented by the general equation.
Double replacement reactions are also called metathesis or double displacement reactions. Many double displacement reactions occur between ionic compounds that are dissolved in water. Solubility rules are used to predict whether some double-replacement reactions will occur.
Solutions of potassium flouride and calcium nitrate are mixed 2KFaq CaNO32aq -- CaF2s 2KNO3aq. Heres an example to better demonstrate the concept. In this reaction an electron from the carbon-carbon double bond of the alkene attacks an incoming molecule XY causing the breakage of the carbon-carbon double bond lefthand diagram and formation of a new bond between one of the alkene carbons and molecule X.
Nicotine replacement therapy NRT may be a helpful tool if youre trying to quit smoking. In a double replacement reaction there are usually 4 compounds in all. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds.
The reaction involves an exchange of cations or anions between the reactants. Write balanced chemical equation for the double-replacement reactions that occur in aqueous solution. C 3 H 8 5 O 2-- 3 CO 2 4 H 2 O.
This product is referred to as a precipitate and the reaction is. This world can be pretty unpredictable but lucky for you predicting products of chemical reactions doesnt have to be. Double Replacement Reactions and Gas Laws - Students will understand how a change a gas system of pressure volume or temperature affects the overall system.
Double replacement reaction. AgNO 3 NaCl --- Silver nitrate sodium chloride silver chloride sodium nitrate AgNO 3 NaCl --- AgCl NaNO 3 1 CaOH 2 H 3 PO 4--- 2 K 2 CO 3 BaCl 2--- 3. Learn about the different kinds of NRT products and which may be right for you.
Double replacement reactions fall into 3 general groups. Double replacement reactions are also called double replacement reactions double displacement reactions or. Science Chemistry library Chemical reactions and stoichiometry Types of chemical reactions Double replacement reactions Definition and examples of double replacement reactions.
The replacement is usually similar to the original object. DOUBLE REPLACEMENT PRACTICE REACTIONS For each reaction predict the products and balance the equation. An example of a double replacement reaction is the reaction between silver nitrate and sodium chloride in water.
K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. In this video learn my quick chemist. Watch the best videos and ask and answer questions in 225 topics and 28 chapters in Chemistry.
Molecular complete ionic and net ionic equations. Molecular complete ionic and net ionic equations. Examples of double displacement.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Double replacement reaction.

Double Replacement Double Displacement Reaction

Single Replacement Reaction Definition And Examples
Double Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Double Replacement Reaction Definition And Examples
What Is A Double Replacement Reaction In Chemistry Socratic

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com
0 comments
Post a Comment